Technology
Stemgenics Non-Integrating Multi-Functionalized Activated NanoParticles (NIMFA-NP) are well suited for precise regulation of intracellular signaling pathways and targeted control of cell fate
Stemgenics Major Therapeutic Focus Areas of Interest
Cell and tissue rejuvenation and longevity
Multi-specific nanoparticles functionalized with different bioactive molecules like telomerase, an enzyme that extends the length of telomeres shortened with age, resulting in resetting the biological clock of the cell and renewing the life cycle
Regenerative medicine
Multi-specific nanoparticles functionalized with peptides, proteins and mRNA turn on or off expression of cell lineage-specific genes and trigger Direct Reprogramming of patients’ cells into
- functional hepatocytes - NASH, cirrhosis, liver fibrosis, etc.,
- beating cardiomyocytes - myocardial infarction, cardiac fibrosis, etc.,
- dopaminergic and other specialized neurons – neurodegenerative diseases
- Direct cell reprogramming using hepatogenic, cardiogenic, neurogenic or other cell-type specific multi-functionalized nanoparticles is an attractive and preferred alternative to pluripotent stem cells that are known to trigger tumorigenesis.
Oncology
Multi-specific nanoparticles functionalized with siRNAs and mRNA inhibit expression of abnormal genes and restore expression of silenced tumor suppressor genes
- Hepatocellular carcinoma
- Glioblastoma
-
mRNA-based vaccines and anti-viral therapeutics
Vaccines:
Multi-specific nanoparticles functionalized with one or more viral gene-specific mRNAs induce expression of one or more viral proteins and activate immune responseAnti-viral therapeutics:
Multi-specific nanoparticles functionalized with two or more viral gene-specific siRNA knock down expression of viral-specific genes preventing viral particle reassembly, viral multiplication and infection
Stemgenics multi-functionalized nanoparticles rapidly within minutes penetrate through cell membrane and enter the cytoplasm and the cell nuclei to reprogram mature human cell into a new cell type of interest
The multi-functionalized nanoparticles exhibit nearly 100% efficiency in cytoplasmic and nuclear delivery of the cargo and demonstrate virtually no cytotoxicity as determined by flow cytometry analyses
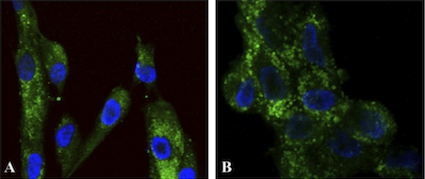 Figure 2. Functionalized nanoparticles targeted to cell cytoplasm.
Two different types of primary
human fibroblast cells (A) and (B) were treated for 30 minutes with fluorescent nanoparticles
functionalized to target cell cytoplasm, washed and stained with DAPI (blue fluorescence
showing nuclei). The green fluorescence shows localization of the functionalized nanoparticles
in the cell cytoplasm.
Figure 2. Functionalized nanoparticles targeted to cell cytoplasm.
Two different types of primary
human fibroblast cells (A) and (B) were treated for 30 minutes with fluorescent nanoparticles
functionalized to target cell cytoplasm, washed and stained with DAPI (blue fluorescence
showing nuclei). The green fluorescence shows localization of the functionalized nanoparticles
in the cell cytoplasm.
Stemgenics multi-specific nanoparticles are highly efficient in delivering bioactive molecules into the cytoplasm of adherent and non-adherent hematopoietic cells
Imaging of human cells treated with nanoparticles functionalized with cytoplasmic targeting elements and labeled with a green fluorescent dye demonstrates that functionalized nanoparticles exhibit nearly 100% efficiency in penetrating through plasma membrane and entering the cytoplasm of the cells. Representative high-resolution images of the cells with cytoplasmic localization of functionalized nanoparticles are depicted in Figure 2 (human fibroblasts) and Figure 3 (human hematopoietic cells).
Fluorescently (FITC) labeled nanoparticles multi-functionalized with cell reprogramming polypeptide molecules, cytoplasmic and nuclear targeting elements effectively enter the cells and concentrate in the nucleus of treated cells. Representative high-resolution images of fluorescently labeled nanoparticles in live cells (Figure 4) or fixed cells (Figure 5) demonstrate virtually 100% efficiency in intracellular delivery of proteins of interest into the cell nuclei using Stemgenics multi-functionalized nanoparticles.
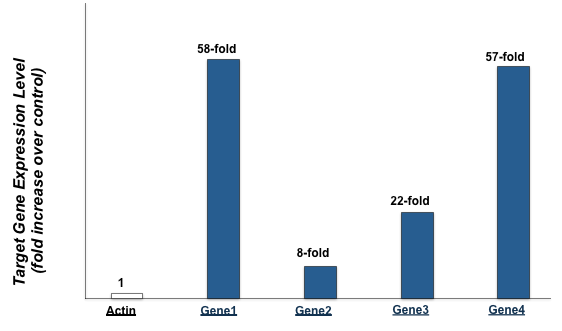 Figure 6. Protein-functionalized nanoparticles trigger robust expression of downstream target
genes in primary human fibroblasts.
Figure 6. Protein-functionalized nanoparticles trigger robust expression of downstream target
genes in primary human fibroblasts.
To demonstrate that nanoparticles multi-functionalized with bioactive protein not only enter the nucleus but also modify expression of target genes, real-time RT-PCR analysis was used to analyze gene expression of actin and four target genes in control cells and cells treated with functionalized nanoparticles. The real-time RT-PCR results depicted in Figure 6 demonstrate that while actin expression was unchanged, the target genes were overexpressed 58, 8, 22, and 57 fold.
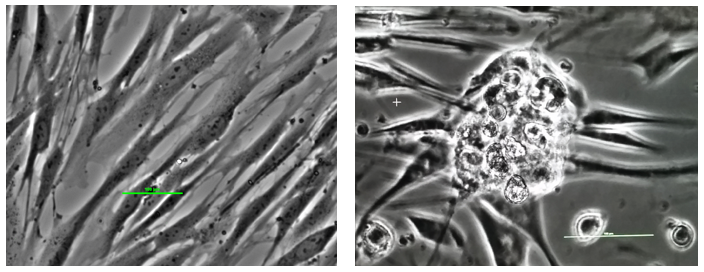 Figure 7. Reprogramming somatic cells into stem cells. The left panel shows fibroblast cells (skin cells) in culture: long narrow cells. The panel on the right shows reprogrammed stem cells: an early stage colony.
Figure 7. Reprogramming somatic cells into stem cells. The left panel shows fibroblast cells (skin cells) in culture: long narrow cells. The panel on the right shows reprogrammed stem cells: an early stage colony.
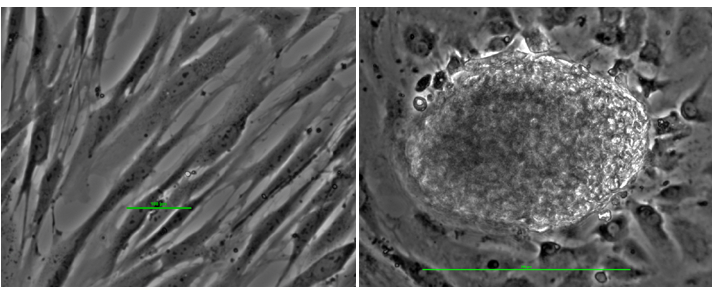 Figure 8. Reprogramming somatic cells into stem cells. The left panel shows fibroblast cells (skin cells) in culture: long narrow cells. The panel on the right shows reprogrammed stem cells: a late stage colony of 200 microns.
Figure 8. Reprogramming somatic cells into stem cells. The left panel shows fibroblast cells (skin cells) in culture: long narrow cells. The panel on the right shows reprogrammed stem cells: a late stage colony of 200 microns.
Culturing mature human cells with cell reprogramming nanoparticles functionalized with bioactive molecules produces early stage stem cell colonies of 100 microns as shown in Figure 7 and late stage stem cell colonies of 200 microns as shown in Figure 8.
Human primary fibroblasts (A) were reprogrammed into human pluripotent stem cells (niPSC) using nanoparticles multi-functionalized with Oct4, Sox2, Nanog and Lin28 transcription factors in complete absence of DNA and characterized by morphology, growth, and presence of characteristic pluripotency markers.
Intracellular delivery of gene-specific siRNAs with Stemgenics NIMFA-NP
Fluorescence images of control and treated cells demonstrate 100% efficiency in intracellular delivery of gene-specific siRNA into the cell cytoplasm using Stemgenics multi-functionalized nanoparticles.
NIMFA-NP functionalized with gene-specific siRNA are highly efficient in knocking down target gene expression in human cells
Stemgenics NIMFA-NPs functionalized with 75 picomoles (pmol) of gene-specific siRNA virtually abolish expression of a target gene as determined by RT-PCR.
Highly efficient intracellular delivery and robust expression of gene-specific mRNA using Stemgenics lipid-free multi-functionalized nanoparticles.
Gene-specific mRNA generated in-house from cDNA was further modified and functionalized onto Stemgenics nanoparticles. Primary human cells treated with mRNA-functionalized nanoparticles were cultured overnight, fixed, and fluorescent images acquired using high resolution fluorescence microscope.

